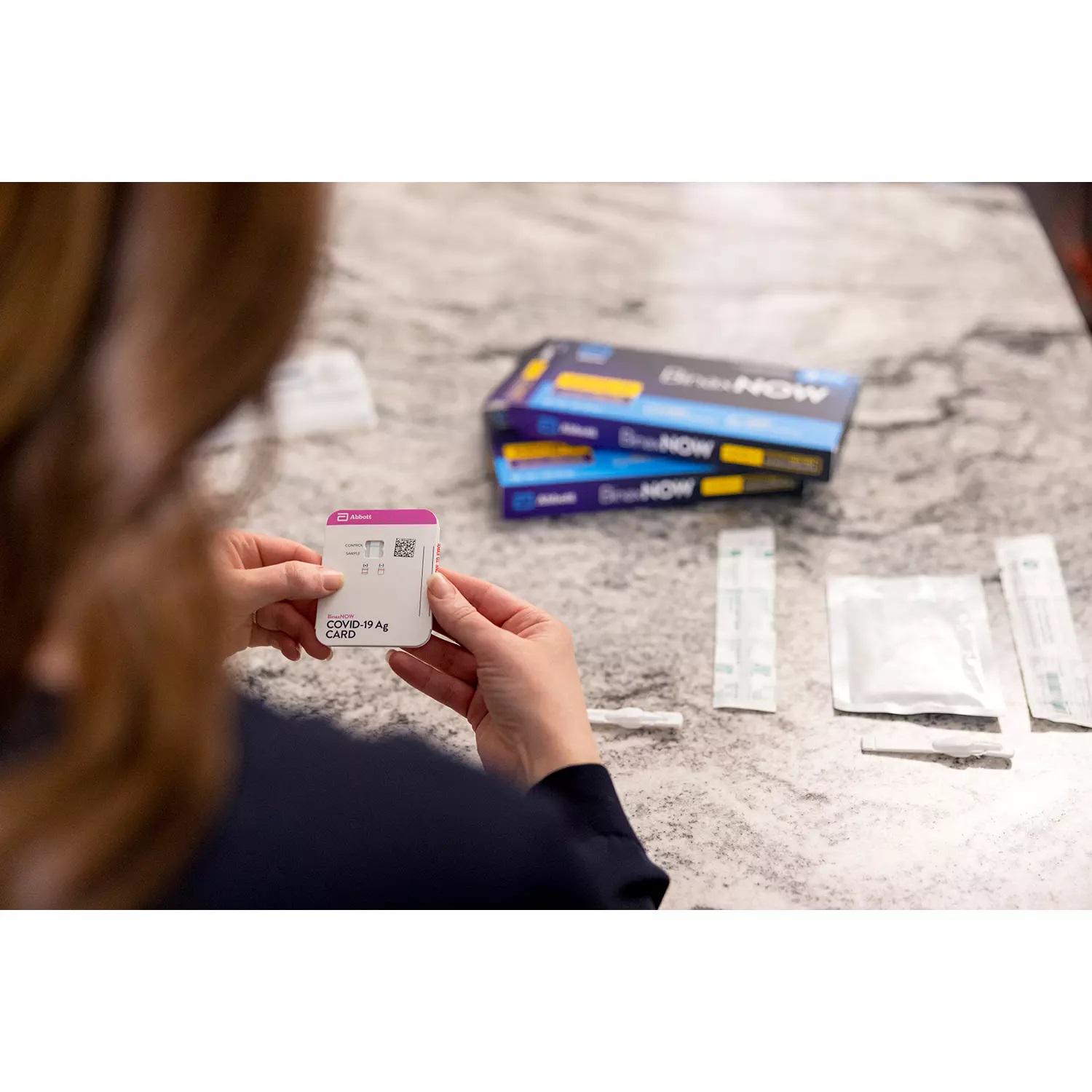
At-home COVID-19 testing kits are now available over-the-counter at some CVS stores, the Rhode Island-based retailer said Monday. "The OTC testing options are not covered by insurance and are not meant to diagnose acute COVID-19 infection or test the efficacy of COVID-19 vaccination," the retailer said. It will be in select locations in Massachusetts and Rhode Island the week of April 19, with increasing availability on CVS.com and in most CVS Pharmacy locations by the end of May.The Abbott BinaxNOW test also provides results in 15 minutes. "The box contains two tests which should be administered twice over three days with at least 36 hours between tests," CVS said. The test is currently in select stores in Massachusetts, Rhode Island, Connecticut and Alabama. The Ellume COVID-19 Home Test uses a mid-turbinate nasal swab to detect certain proteins of the virus known as antigens.
The Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms. In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples. The Ellume COVID-19 Home Test uses an analyzer that connects with a software application on a smartphone to help users perform the test and interpret results. Results are delivered in as little as 20 minutes to individuals via their smartphone.
The mobile application requires individuals to input their zip code and date of birth, with optional fields including name and e-mail address, and reports the results as appropriate to public health authorities to monitor disease prevalence. Ellume expects to produce more than three million tests in January 2021. This test is authorized for non-prescription home use with self-collected observed direct anterior nasal swab samples from individuals aged 15 years or older or adult collected anterior nasal swab samples from individuals aged two years or older. The BinaxNOW COVID-19 Ag Card 2 Home Test is to be performed only with the supervision of a telehealth proctor. This test is authorized for non-prescription home use with self-collected direct anterior nasal swab samples from individuals aged 15 years or older or adult collected anterior nasal swab samples from individuals aged two years or older.
The Lucira CHECK IT Test Kit fits in the palm of a hand, extracts genetic material from the virus and amplifies it similar to PCR lab tests. Each Lucira test kit contains everything needed to run one COVID-19 test. Users get the test device, two AA batteries, sample vial, swab and simple instructions.
The batteries are inserted in the device and the sample vial is placed in the test unit. The user then opens the test swab packet and rotates the swab in each nostril five times. The swab is then stirred in the sample vial, which is then gently pressed into the test unit to start the test. The "ready" light will blink until a "positive" or "negative" green light is illuminated within 30 minutes. For guidance on care and public health reporting, people can use Lucira's text based, secure LUCI portal to receive a result verification back on their phone while at the same time transmitting their result to the relevant public health authorities.
It's important to remember that rapid antigen tests serve a different purpose than PCR testing, which is considered the gold standard even though it isn't 100% accurate. Rapid tests are designed to identify cases with a high enough viral load in the nasal passage to be transmissible – not to diagnose all COVID-19 cases. The Abbott BinaxNOW rapid antigen test may only detect 85% of the positive cases detected by PCR tests. But the key is that published studies found that they detect over 93% of cases that pose a transmission risk, which is what matters most for getting the pandemic under control.
Ellume correctly identifies 95% of all positive cases, and Quidel QuickVue accurately identifies 85%. All three tests correctly identify upwards of 97% of all negative cases, regardless of symptoms. They have been authorized by the FDA under an emergency use authorization. The PCR tests available at doctor's offices and pharmacies are "the gold standard" for accuracy, Troisi said. The downside is results take longer — a day or two or sometimes more — to produce, while rapid antigen tests take around 15 minutes. Rapid tests, meanwhile, are not as sensitive so false negatives are possible.
Troisi also recommended taking two rapid tests in the event a test is positive, as false positives are possible but less likely to happen twice. The Ellume COVID-19 Home Test has not been FDA cleared or approved; but has been authorized by the FDA under an emergency use authorization. If you get a negative result from a rapid test, it means you are currently very unlikely to be infectious. A viral load that is too low to be detected by rapid antigen tests is almost surely too low to be transmissible.
The tests don't detect 100% of infectious cases, so it's possible for a small number to evade detection or for some cases to become infectious within hours after the test. And, if you have symptoms or a known exposure, it's a good idea to do a follow-up rapid antigen or PCR test just in case the first test was a false negative. Abbott BinaxNOW COVID-19 Antigen Self-Test $23.99The BinaxNOW COVID-19 Antigen Self Tests have not been FDA cleared or approved. Ellume COVID-19 Home Test Kit $38.99The Ellume COVID-19 Home Test has not been FDA cleared or approved; but has been authorized by the FDA under an emergency use authorization. This home test is authorized for nonprescription home use with self-collected direct anterior nasal swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older. According to the manufacturer's instructions for use, people using the test should test themselves twice with at least 36 hours between tests.
The test can be used on children as young as two years old when samples are collected by an adult and for all people aged 15 years or older. Individuals who test negative should continue to stay cautious, including following social distancing, hand washing and wearing a mask. Centers for Disease Control and Prevention guidelines, which is to communicate your results to your healthcare provider, who is responsible for reporting your test results to the state health department.
Some accuracy issues can be averted by taking these tests several times a week, if people can find and afford them. Repeat testing is what the FDA now recommends for people using over-the-counter tests as asymptomatic screens. The two-pack BinaxNOW tests, for instance, are meant to be taken fewer than three days apart; some models advocate that rapid tests be taken almost daily. "It's clear that testing, particularly rapid testing, will be needed to manage this next phase of the pandemic," John Koval, Abbott's director of public affairs for rapid diagnostics, told me. Rapid antigen tests, like the polymerase chain reaction tests, check for active infection of COVID-19. PCR tests, which require a lab to detect the genetic material of the coronavirus, are the gold standard for COVID-19 testing.
Point-of-care tests are in high demand in order to facilitate rapid care decisions for patients suspected of SARS-CoV-2. Positive percent agreement between Cue COVID-19 and reference SARS-CoV-2 test was 91.7% (22/24); or 95.7% (22/23) when one patient with no tie-breaker method was excluded. Negative percent agreement was 98.4% (239/243), and there were 25 (8.6%) invalid or canceled results. The Cue COVID-19 Test demonstrated very good positive and negative percent agreement with central laboratory tests and will be useful in settings where accurate POC testing is needed to facilitate management of patients suspected of COVID‑19. Five at-home, over-the-counter COVID-19 tests have received FDA authorization. Three are rapid antigen tests , and the other two are molecular-based tests .
None of these tests require samples to be sent to a lab – you can get results in your own home. Scott Koepsell, MD, PhD, is the medical director of the University of Nebraska Medical Center main testing lab. Lucira is a medical technology company focused on the development and commercialization of transformative and innovative infectious disease test kits.
Lucira's testing platform produces lab quality molecular testing in a single-use, consumer-friendly, palm size test kit powered by two AA batteries. Lucira designed its test kits to provide accurate, reliable and on-the-spot molecular test results anywhere and at any time. The LUCIRA CHECK IT and LUCIRA COVID-19 All-In-One Test Kit are designed to provide a clinically relevant COVID-19 result within 30 minutes from sample collection. A simple solution for COVID-19 infection detection, with rapid results in the convenience of your home. Authorization for self-testing without the need to ship samples to a lab or for a prescription from your healthcare provider.
Simply test yourself twice within 3 days, with at least 36 hours between tests. This test does NOT meet the CDC testing requirements to enter the U.S. when returning from a trip abroad. Because it uses a phone app, you'll need an internet connection to use Ellume, which involves communication between your phone and the kit via Bluetooth.
An advantage of the app is that it provides good directions and an electronic receipt for your test—the kind you can show to a school or employer. The others I tried didn't have a paper trail, so there's no proof you took the test. Of the three tests I tried, Ellume's was the only one that isn't entirely private.
The app warns that it will share "certain information with public health authorities." That information turns out to include your birthday, your zip code, and your test result. The company says the data helps health agencies track the pandemic and report infection levels. The UK government started giving away covid antigen tests for free, by mail and on street corners, on April 9, saying it wants people "to get in the habit" of testing themselves twice a week as social distancing restrictions are eased. Along with vaccines, free tests are part of that nation's plan to quash the virus.
Later, though, a leaked government memo said health officials were privately worried about a tsunami of false positives. This test is authorized for use with direct anterior nasal swab samples from individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested twice over three days with at least 36 hours between tests. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 , 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This test is authorized for use at the Point of Care , i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Rapid antigen tests have a big advantage over lab-based PCR testing in terms of speed and convenience. Getting results in 15 minutes rather than waiting a day or more for PCR test results means it's possible to identify COVID-19 cases right away and take precautions to prevent transmission. Having rapid testing available over-the-counter means that a lot more people will get tested since the test is easy to perform and far more convenient than PCR testing. So rapid tests can catch a lot more COVID-19 cases overall than relying only on PCR testing. For months, health experts have stressed the need for fast, widespread home testing so that people can screen themselves and avoid contact with others if they have an infection.
But the vast majority of tests still require a nasal swab performed by a health worker that must be processed at high-tech laboratories. About 25 tests allow people to collect their own sample at home— a nasal swab or saliva — but then that's shipped to a lab. Download and open the free Ellume COVID-19 Home Test app on your phone.
The app will guide you through the test including an integrated information video and step-by-step instructions. There are 7 simple steps to perform the test, including our user-friendly, patented nasal swab. Your sample is analyzed and your result is displayed on your phone after 15 minutes. The Ellume COVID-19 Home Test complies with CDC reporting requirements and automatically reports the required data to health authorities through our secure encrypted, HIPAA compliant, cloud connection. Thanks to these bells and whistles, and a special swab, Ellume has a higher accuracy rate for spotting covid than other antigen tests, missing only one in 20 infections, according to the company. The drawback is that it is 50% more likely than other tests to falsely inform you that you are positive for covid-19 when you are not.
Everlywell's Covid test collection kit is designed for those 18 years old and above. You collect a nasal swab and mail the sample to the lab, which performs a PCR test and delivers your digital results within 24 to 28 hours of the sample arriving at the lab. Telehealth consultants are available at no extra cost to guide you if your result is positive. The FDA reminds patients that all tests can experience false negative and false positive results. Individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and experience COVID-like symptoms should follow up with their health care provider as negative results do not preclude an individual from SARS-CoV-2 infection. Advocates of screening with inexpensive rapid antigen tests say home tests are an important step to give consumers the option of testing themselves without visiting a doctor or telehealth provider – extra steps that take time and cost money. The LUCI Pass was developed to support Lucira's over-the-counter test kit and is unique to Lucira. Users simply text a short code to access LUCI, and then go through a simple sequence of steps including scanning their test result to receive a LUCI Pass and verified test to their phone.
Results are also transmitted to the required public health authorities. Each single-use test kit contains everything needed to conduct one COVID-19 test. It can detect a positive result in as few as 11 minutes or confirm a negative result within 30 minutes. It was designed and tested extensively for individuals to use independently and does not require a physician's prescription or telehealth / supervised assistance. A large-scale review of 68 studies published March 24 found that rapid antigen tests identify about 72 percent of people with symptoms and only 58 percent of those without symptoms. Antigen tests are most accurate when used within the first week after symptoms develop, the review found.
Additionally, Nachman noted that most at-home test kits come with two tests and recommend that you perform multiple tests a few days apart — this is called serial testing, according to the Centers for Disease Control and Prevention. Especially in asymptomatic adults, on the first day you perform an at-home test, it might not be able to detect the virus and your result may be negative — this could be false. Thus, the CDC states "you could test positive later during your illness," emphasizing why serial testing is recommended. Amazon's Covid test collection kit allows you to perform a nasal swab and mail the sample to Amazon's lab with the included prepaid UPS next-day shipping. You can expect to receive results within 24 hours of your sample arriving at the lab. The best type of diagnostic Covid test is the Polymerase Chain Reaction test, according to Omai Garner, PhD, chief of clinical microbiology for UCLA Health.
No PCR test is approved for at-home testing, meaning "the most accurate Covid test cannot be done entirely at home," he said. At-home testing kits are not as accurate as PCR tests done in professional labs because at-home testing — sometimes referred to as "rapid tests" — require a higher amount of virus in a sample in order to detect a positive result. If you test too early, there may only be low levels of the virus present in the sample, which could lead to an inaccurate result.
Ileana Geiser, certified pharmacy technician, reads the directions on a BinaxNOW COVID-19 Antigen self test after a customer asked a question about the tests over the phone at Magnolia Pharmacy Monday, Aug. 16, 2021 in Magnolia. Demand for quick COVID-19 testing appeared to outpace supply in Houston last week as the delta variant spread rapidly. Testing slots at CVS and Walgreens were booked up several days out and you'd be lucky to find a store with any home testing kits left.
Manufacturers of BinaxNOW Self Tests said demand for its products is increasing as cases rise. Ileana Geiser, certified pharmacy technician, returns a BinaxNOW COVID-19 Antigen self test to the shelf after a customer asked a question about the tests over the phone at Magnolia Pharmacy Monday, Aug. 16, 2021 in Magnolia. Food and Drug Administration issued an emergency use authorization for the first over-the-counter fully at-home diagnostic test for COVID-19.
The Ellume COVID-19 Home Test is a rapid, lateral flow antigen test, a type of test that runs a liquid sample along a surface with reactive molecules. The test detects fragments of proteins of the SARS-CoV-2 virus from a nasal swab sample from any individual 2 years of age or older. It's important to have a plan for what to do based on the test results. If you get a positive result, immediately take precautions to slow transmission such as self-isolating, letting close contacts know about the test result and reporting the case to health authorities. Less than 3% of negative cases receive false positives, but a second rapid test the following day or a PCR test can provide further confirmation if needed.
Of the nine FDA authorized COVID-19 tests, three are BinaxNOW COVID-19 rapid antigen tests, available as the over-the-counter, prescription and proctor-supervised, which provide results in 15 minutes. The nasal-swab test costs around $20 for a pack of two, depending on the retailer. Like other tests that scan for proteins, FDA officials noted that Ellume's test can deliver a small percentage of false positive and false negative results. People who get a negative result but have coronavirus symptoms should follow up with a health professional, the agency said. This home test has not been FDA cleared or approved, but has been authorized by the FDA under an Emergency Use Authorization for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.




























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.